find number of atoms|atom number definition : Baguio The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom components - protons, neutrons, and electrons (or .
9 de out. de 2023 · Renato Albani, Afonso Padilha, Diogo Portugal, Danilo Gentili, Fábio Rabin, entre outros humoristas consagrados tocaram nesses assuntos, claro, de forma .
0 · what do atomic numbers represent
1 · how to find atomic no
2 · element that has 12 protons
3 · atomic weight vs mass number
4 · atomic number is represented by
5 · atomic number how to find
6 · atom number definition
7 · atom number calculator
8 · More
Resultado da This captivating game is a non-stop party of fun that is over-flowing with rewards. Take a break from the hustle and bustle and explore the tents while enjoying a refreshing and frosty beer. Bier Haus Riches is a 3x1 slot that expands into a 3x3 layout every time 2 Scatters land. When the reels .
find number of atoms*******This chemistry video tutorial explains how to calculate the number of atoms in molecules and compounds. It also explains how to convert moles to atoms and g.In 1 mole of a substance, the number of atoms is N A or 6. 023 × 10 23 atoms. Multiply the given moles with N A to find the total number of atoms. Example 1: To calculate the .
If you have a sample that contains atoms of a single element, you can find the number of atoms by weighing it. To calculate the number of atoms in a formula, the weight of a sample, its atomic mass from the periodic table and a constant known as Avogadro’s number are .
atom number definitionKnowing the mass of an atom, you can calculate the number of atoms in any mass of matter. Use our number of atoms calculator to quickly and easily calculate the total . The atom calculator is a tool for calculating the atomic number and the mass number based on the number of atom components - protons, neutrons, and electrons (or . One mole of anything, including atoms, is 6.022xx10^23 (Avogadro's number) of them. Usually you will have a given mass of an element. There are two basic . To find the number of atoms in a molecule (sometimes called atomicity) we need to determine the number of each type of element. For each element the subscript, the number .
The number of moles in a system can be determined using the atomic mass of an element, which can be found on the periodic table. This mass is usually an . How do you work out the number of atoms and molecules using the number of moles and Avogadro’s constant? Understanding the link between the mole, .Step 2: Calculate the number of atoms The number of atoms= number of moles × N A Since CO 2 is triatomic. i.e 1 molecule of CO 2 contain 1 atom of C and 2 atom of O The total number of atoms = 0. 5 × 3 × 6. 023 × 10 23 = 9. 0345 × 10 23 atoms. C = 0. 5 × Number of atoms = 2 moles x 6.02214076 × 10 23 atoms/mole ≈ 1.2044 × 10 24 atoms. Another way of calculating the number of atoms is through a two-step process: moles to grams and then grams to atoms. This method requires the atomic mass of an element, which you get from the periodic table. For iron (Fe), its molar mass is about .
find number of atoms One mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The number 6.022 × 10²³ is known as Avogadro's number or .The mole is defined as the amount of substance that contains the number of carbon atoms in exactly 12 g of carbon-12 and consists of Avogadro’s number (6.022 × 10 23) of atoms of carbon-12. The molar mass of a substance is defined as the mass of 1 mol of that substance, expressed in grams per mole, and is equal to the mass of 6.022 × 10 23 atoms, .The units for molar mass are grams per mole or g/mol. 1.00mol of carbon-12 atoms has a mass of 12.0g and contains 6.022 × 1023 atoms. 1.00 mole of any element has a mass numerically equal to its atomic mass in grams and contains 6.022 × 1023 particles. The mass, in grams, of 1 mole of particles of a substance is now called the molar mass . The number of atoms is just a number; therefore, it is dimensionless, i.e., it does not have any units. Rearrange the formula to find Avogadro's constant: number of atoms / moles = Avogadro's number So, on the left-hand side, we have no units / moles , which can be expressed as mol -1 .
8.1: Counting Atoms by the Gram. In chemistry, it is impossible to deal with a single atom or molecule because we can't see them or count them or weigh them. Chemists have selected a number of particles with which to work that is convenient. Since molecules are extremely small, you may suspect this number is going to be very large and you are .One mole is 6.022 x 10 23 of the microscopic particles which make up the substance in question. Thus 6.022 x 10 23 Br atoms is referred to as 1 mol Br. The mole is an amount unit similar to familiar units like pair, dozen, gross, etc. It provides a specific measure of the number of atoms or molecules in a bulk sample of matter.And since each simple cubic unit cell has one atom at each of its eight “corners,” there is 8 × 1 8 = 1 8 × 1 8 = 1 atom within one simple cubic unit cell. Figure 4.1.4 4.1. 4: A simple cubic lattice unit cell contains one-eighth of an atom at each of its eight corners, so it contains one atom total. Most metal crystals are one of the .
Can you solve this real interview question? Number of Atoms - Given a string formula representing a chemical formula, return the count of each atom. The atomic element always starts with an uppercase character, then zero or more lowercase letters, representing the name. One or more digits representing that element's count may follow if the count is .
How Many Atoms Exist in the Universe? The universe is vast. Scientists estimate there are 10 80 atoms in the universe. Since we can't go out and count each particle, the number of atoms in the .
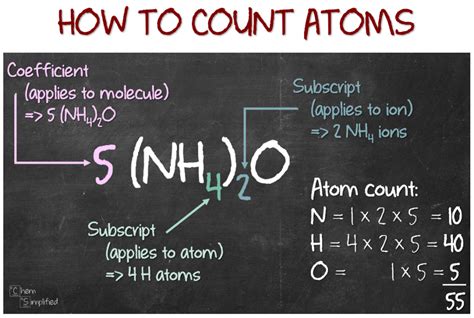
The atoms to mole are totally based on the Avogadro’s number which is a numerical constant. So: 6.0221415E+23 atoms = 1 Mole atoms= 1 Mole/6.0221415E+23 The general atoms to moles formula is : Number of atoms= Mole/Avogadro’s Number Where: 6.0221415E+23= 6.022140857 x 10^23.

Step 4: Find how many atoms are in the number of moles we found in Step 3 by multiplying the number of moles by Avogadro's number. We found that in 2g of Tin(Sn), there are {eq}1.685 \times 10^{-2 .Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is .Avogadro's Constant. One mole of oxygen atoms contain s 6.02214179 ×1023 6.02214179 × 10 23 oxygen atoms. Also, one mole of nitrogen atoms contain s 6.02214179 ×1023 6.02214179 × 10 23 nitrogen atoms. The numb er 6.02214179 ×1023 6.02214179 × 10 23 is cal led Avogadro's number ( NA N A) or Avogadro's constant, after the 19th century .A unit cell is the smallest representation of an entire crystal. All crystal lattices are built of repeating unit cells. In a unit cell, an atom's coordination number is the number of atoms it is touching. The simple cubic has a sphere at each corner of a cube. Each sphere has a coordination number of 6 and there is 1 atom per unit cell.Figure 3.3.1 3.3. 1 shows that we need 2 hydrogen atoms and 1 oxygen atom to make 1 water molecule. If we want to make 2 water molecules, we will need 4 hydrogen atoms and 2 oxygen atoms. If we want to make 5 molecules of water, we need 10 hydrogen atoms and 5 oxygen atoms. The ratio of atoms we will need to make any number of water .Avogadro's number (Equation 1.4.2 1.4.2) like any pure number, is dimensionless. However, it also defines the mole, so we can also express NA as 6.02 × 1023 mol–1; in this form, it is properly known as Avogadro's constant. This construction emphasizes the role of Avogadro's number as a conversion factor between number of moles and number of .
web4 de out. de 2023 · Andressa Urach e filho fazem sexo com a mesma mulher. Mari Ávila fez sexo com Andressa Urach e com o filho e filmaker dela, Arthur Urach. Depois de .
find number of atoms|atom number definition